Balanced Equation of Sodium Carbonate and Hydrochloric Acid
The chemical equation is given below. Calculate the number of moles of sodium hydroxide used use balanced equation to give moles of HCl 1 mol NaOH.

35 Answer The Following Questions 1 State The Reaction Of Sodium Carbonate And Sodium Hydrogen Brainly In
Memorize flashcards and build a practice test to quiz yourself before your exam.

. Predict the names of the products write the balanced chemical equation ionic net ionic equations and spectator ions for the following two metathesis reactions. Furthermore what happens when you mix NaHCO3 and HCl. Acid-Base neutralization Combustion Write balanced equations for each of the following examples.
Uses of Sodium Oxide Na 2 O. Aluminum and hydrochloric acid yields aluminum chloride and hydrogen. Write a balanced chemical equation for this reaction.
Na2O 2HCl 2NaCl H2O. 0300 mol dm3 sodium hydroxide solution. N Put a drop of each of the above solutions on a watch-glass one by one and test with a drop of the indicators shown in Table.
AB C A CB. HCl reacts with sodium carbonate chemical formula. Copper is unable to displace hydrogen from non-oxidizing acids for instance hydrochloric acid or diluted sulphuric acid.
Na Cl 2 2NaCl. What Is a Balancing Chemical Equations Worksheet. Work out number of moles of hydrochloric acid left in 100 cm3 1.
Sodium oxide reacts with carbon dioxide to form sodium carbonate. But it is useful in balancing large redox equations. Calcium hydroxide and phosphoric acid yields calcium phosphate and water.
Here an acid and a base Hydrochloric acid and Sodium Hydroxide react in a neutralization reaction to produce Sodium ChlorideCommon Salt and water as the products. How to balance the reaction from redox method. Calcium carbonate yields calcium oxide and carbon dioxide.
When copper gets heated with concentrated. The chemical equation for this reaction is given below. A REDuction-OXidation reaction is a reaction in which there is a transfer of electrons between chemical species.
-NaCl - CaCl2 -FeCl3 -BaCl2 - PbCl2 The equation 2Als 3Br2l 2AlBr3s is an _____ reaction. Sulfur and oxygen yields sulfur trioxide. Calculate the number of moles of sodium hydroxide used use balanced equation to give moles of HCl 1 mol NaOH.
Redox method will take much time. The equation is now balanced and it is interpreted to mean that 2 molecules of AlOH 2. Na2CO3 one of two reactions will take place depending on the relative quantities of each chemical.
2 HCl 2 Na 2 NaCl H 2. M2 balanced equation H 3O in place of H 2. Metal oxide non-metal oxide K2O SO3 K2SO4 potassium sulfur potassium oxide trioxide sulfate CaO CO2 CaCO3 calcium carbon calcium oxide dioxide carbonate c.
In other words we can say that the copper does not react with the diluted sulphuric acid. Hydrogen and nitrogen monoxide yields water and nitrogen. What is the balanced equation for hydrochloric acid and sodium carbonate.
Hydrochloric acid HCl sulphuric acid H 2 SO 4 nitric acid HNO 3 acetic acid CH 3 COOH sodium hydroxide NaOH calcium hydroxide CaOH 2 potassium hydroxide KOH magnesium hydroxide MgOH 2 and ammonium hydroxide NH 4 OH. Calcium and oxygen yields calcium oxide. Start studying the chem quiz 7 flashcards containing study terms like Which of the following ionic compounds is insoluble in water.
AB A B sodium bicarbonate s carbon dioxide g sodium carbonate s water l 3. A B AB Sodium s chlorine g sodium chloride s 2. Magnesium reacts with hydrochloric acid according to the equation.
2Nas 2HClaq - 2NaClaq H 2 g Step 2. Only compounds that are aqueous are split into ions 2Nas. Mgs 2 HClaq MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to.
Write the ionic equation for the word equation. Balanced chemical equation of Na Cl 2 reaction. Sodium oxide reacts with some acids such as hydrochloric acid to form sodium chloride and water.
Combining an acid with. It is easy to balance this reaction by inspection method than redox method. Work out number of moles of hydrochloric acid left in 100 cm3 1.
When hydrochloric acid chemical formula. Let us consider the example of an. What is the percentage of CaCO3 by mass in the tablet amount conc x vol 030 x 00111 0.
IGNORE carbon dioxide carbonic acid calcium carbonate hydrogen chloride for hydrochloric acid correct formulae 1 ii. Write the equation and balance it. It is an active flux.
Sodium chloride Fe S FeS ironII sulfide b. Barbie pegasusun sihri tek parça türkçe dublaj izle Not defteri lisans Eylül barbie pegasusun sihri izle. -oxidation-reduction and synthesis -oxidation-reduction only -synthesis.
0300 mol dm3 sodium hydroxide solution. However it does react with the concentrated sulphuric acid because sulphuric acid in concentrated form is an oxidizing agent. Sodium carbonate hydrochloric a.
Write a balanced ionic equation Ag aq Cl-aq AgCls Example. Question Number Answer Accept Reject Marks 5 c M1- dissolve both leadII nitrate and sodium chloride in water penalise M1 is any other reagents added dissolve one in water 1 M2-. What is the percentage of CaCO3 by mass in the tablet amount conc x vol 030 x 00111 0.
2Na 2 O 3CO 2 2Na 2 CO 3. A balancing chemical equation worksheet is a practice booklet with unsolved and solved chemical equation problems on which students. This is a balanced chemical equation representing the reaction between sodium metal and hydrochloric acid reactants to produce sodium chloride solution and hydrogen gas products.
This equation would be read as two HCl plus two Na yields two NaCl and H two But for equations involving complex chemicals rather than reading the letter and its subscript the chemical formulas are read using IUPAC. Non-metal non-metal C O2 CO2 carbon dioxide N2. Two moles of chlorine gas reacts with one mole of sodium to produce two moles of sodium chloride.
As an example the equation for the reaction of hydrochloric acid with sodium can be denoted. When an aqueous solution of sodium hydroxide NaOH is added to an aqueous solution of chromiumIII nitrate CrNO33 a precipitate of. Sodiums hydrochloric acidaq - sodium chlorideaq hydrogeng Solution.
Aqueous sulfurous acid H2SO3 and aqueous sodium chloride are formed by the reaction of aqueous sodium sulfite Na2SO3 and aqueous hydrochloric acid HCl.

Net Ionic Equation For Na2co3 Hcl Sodium Carbonate Hydrochloric Acid Youtube

Na2co3 Hcl Sodium Carbonate Hydrochloric Acid Youtube

Volumetric Analysis Acid Base Ppt Video Online Download

How To Balance Nahco3 Hcl Nacl Co2 H2o Sodium Bicarbonate Plus Hydrochloric Acid Youtube
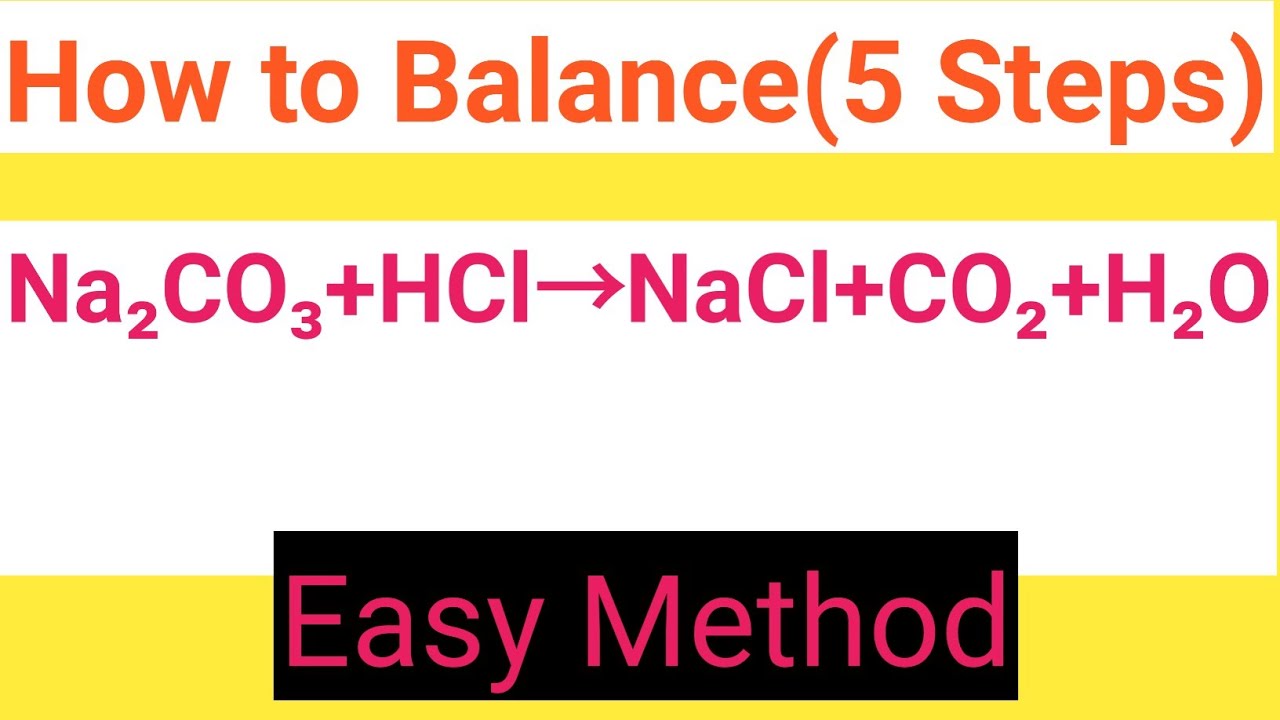
Na2co3 Hcl Nacl Co2 H2o Balanced Equation Sodium Carbonate Hydrochloric Acid Sodium Chloride Carbon Youtube

Sodium Carbonate Hydrochloric Acid Na2co3 Hcl Molecular Equations Net Ionic Equations Youtube

Assertion A Gas Bubbles Are Observed When Sodium Carbonate Is Adde

How To Balance Na2co3 Hcl Nacl H2o Co2 Sodium Carbonate Hydrochloric Acid Youtube

Sodium Carbonate Hydrochloric Acid Na2co3 Hcl Molecular Equations Net Ionic Equations Youtube

Sodium Carbonate Reacts With Hydrochloric Acid Balanced Equation Brainly In

Write A Balanced Chemical Equation For The Reaction Between Sodium Carbonate And Hydrochloric Acid Indicating The Physical State Of The Reactants And The Products
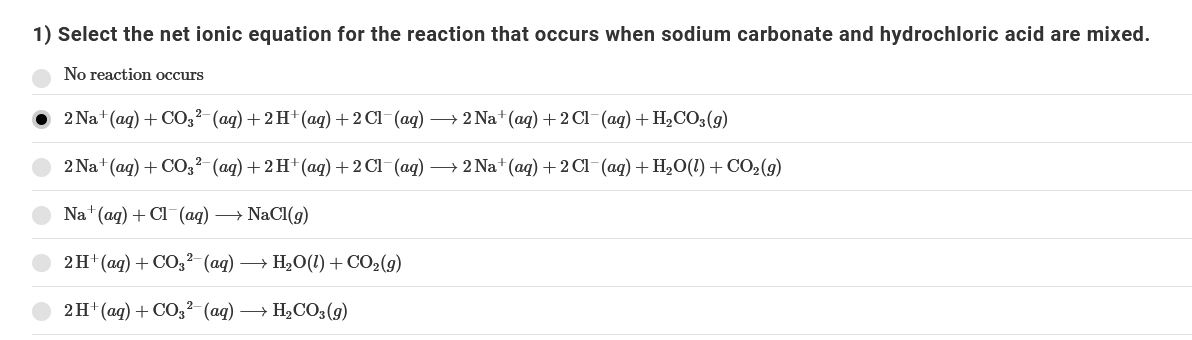
Answered 1 Select The Net Ionic Equation For Bartleby
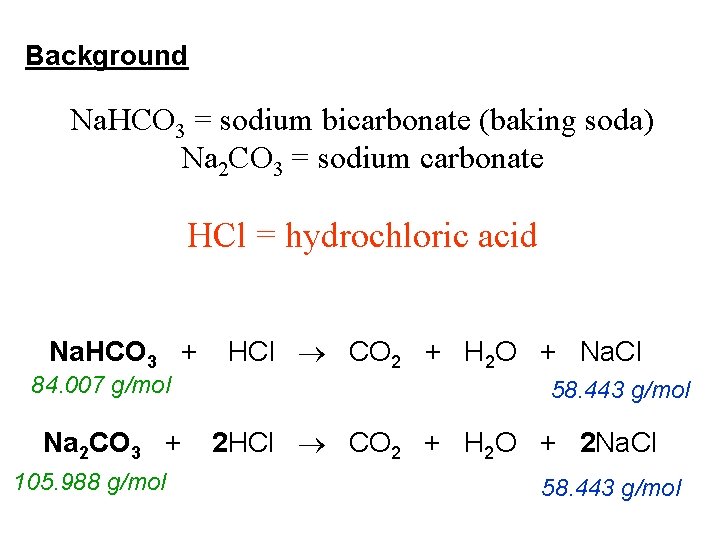
Lab 8 Sodium Carbonate Or Sodium Bicarbonate Objective

Question Video Determining The Products Formed From The Reaction Between Sodium Carbonate And Hydrochloric Acid Nagwa

Sodium Carbonate Hydrochloric Acid Balanced Molecular And Net Ionic Equation Na2co3 Hcl Quizalize

Solved E The Balanced Equation For The Reaction Between Chegg Com

Type Of Reaction For Na2co3 Hcl Nacl H2o Co2 Youtube

Solved Stoichiometry You Need To Plan An Experiment For The Reaction Of Sodium Carbonate With Hydrochloric Acid According To The Balanced Chemical Equation Shown Below What Mass Of Nacl Would Be Produced From
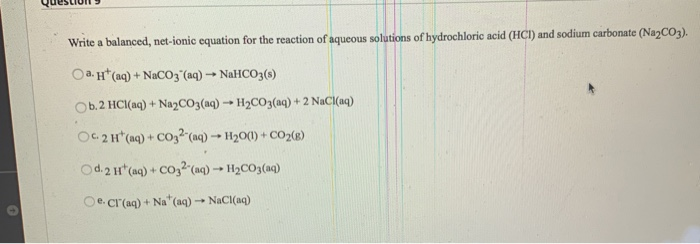
Solved Write A Balanced Net Ionic Equation For The Reaction Chegg Com
Comments
Post a Comment