What Does Graphite and Diamond Have in Common
The chemical composition of the two is exactly the same. Diamond and graphite are both allotropes of carbon.
Diamonds are very valuable as gemstones because they are very pretty and very hard.
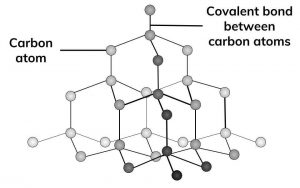
. This makes graphite and diamonds allotropes of carbon along with amorphous which is commonly called soot or carbon black. Both graphite and diamonds are made out of pure carbon. Diamond has a tetrahedral structure and is the hardest material known to man.
Diamond has a tetrahedral structure and is the hardest material known to man. Yet diamond is the hardest mineral known to man 10 on the Mohs scale and graphite is one of the softest less than 1 on the Mohs scale. Both graphite and diamonds are made out of pure carbon.
They both have a giant covalent structure. They are all forms of carbon. Both graphite and diamonds are made out of pure carbon.
Diamonds are the hardest known natural substance and have a hardness of 10. Do diamond and graphite have the same. Both graphite and diamonds are made out of pure carbon.
This makes graphite and diamonds allotropes of carbon along with amorphous which is commonly called soot or carbon black24 thg 4 2017. One bond What do diamonds graphite fullerenes and nanotubes have in. What properties do diamond and graphite have in common.
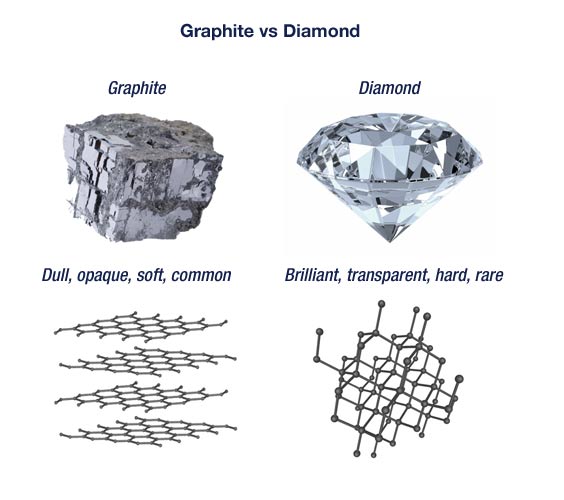
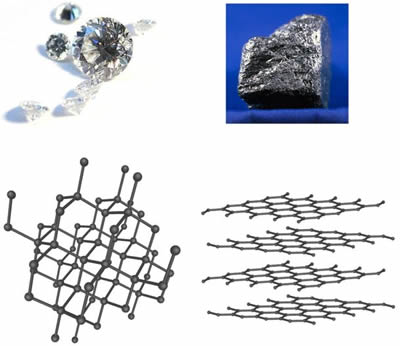
They also crystallize in different crystal systems. The bonding of the atoms in a diamond is much stronger than the bonding in graphite. Diamond in the cubic system and graphite in the hexagonal system.
Diamond and Graphite both are known as the allotropes of carbon. Graphite and diamond are two of the most interesting minerals. Diamond can be found or it.
What Does a Lump of Coal a Pencil Lead and a Diamond have in common. For example diamond is one of the hardest known substances while graphite is one of the softest. These minerals chemically consist of carbon atoms with different physical properties.
Diamond atoms have a rigid 3 dimensional structure with each atom carefully loaded with each other as well as connected to 4 other carbon atoms. They are identical chemically. Both graphite and diamonds are made out of pure carbon.
Both diamond and graphite have a very simple chemical composition. That is that they are both carbon but the crystal lattices are stacked differently. Diamond Diamond is a element and it is very hard to break.
Solve any question of Some p-Block Elements with- Patterns of problems. What do graphite and diamond have in common. What does graphite and diamond have in common.
Diamond has a tetrahedral structure and is the hardest material known to man. This means they are both made up of carbon atoms arranged differently and exist in the same physical state. As both graphite and Diamond are allotropes of carbon the atomic weight of either of them will be equal to the atomic weight of carbon.
What do graphite diamond have in common. This strong network makes diamonds very hard. This is because the carbon atom in both diamond and graphite are regularly arranged to make a crystal.
So the bond in each layer graphene is strong. The chemical composition of the two is exactly the same. This means they are both made up of carbon atoms arranged differently and exist in the same physical state.
Diamond coal graphite coke and buckminsterfullerene are composed primarily of carbon and are insoluble in water. Graphite and diamond are polymorphs of carbon. The only difference between them is the structural placement of the carbon atomBoth graphite and diamond are.
These minerals in general are known to be as polymorphs having the same type. What does diamond coal graphite cokeand buckminsterfullerene have in common. Diamond and graphite are allotropes which means different atomic arrangements of the same elementDiamond and pencil lead graphite are both forms of the element carbon.
What does graphite and diamond have in common. They both have a giant covalent structure. Diamond and graphite are both allotropes of carbon.
What have diamonds graphite and carbon have in common. The chemical composition of the two is exactly the same. Diamond and also graphite are chemically the same both made up of the element carbon however they have entirely different atomic and also crystal frameworks.
The chemical composition of the two is exactly the same. Although graphite and diamond have the same chemical composition their different crystal structures give them very different physical characteristics. Graphite is very soft and has a hardness of 1 to 2 on this scale.
These 3 substances are made up of a common compound ie Carbon. Diamonds are typically clear make excellent abrasives and are good electrical insulators. This means they are both made up of carbon atoms arranged differently and exist in the same physical state.
Graphite is the lead in pencils. They are both pure carbon. What do the minerals graphite and diamond have in common.
Diamond has a linked Carbon structure and that is why it is hard. The common thing in graphite and diamond is their crystalline crystal structure. Graphite collection of graphene layers has higher melting point than diamond because of the partial double bond character of C-C bonds.
Why does graphene have a higher melting point than diamond. Also there are attractive forces between successive layers of graphene increasing the melting. Posted by Sharif Khan on 1st Feb 2021.
What does a dash represent when written between two carbon symbols in a diagram of a chain or ring of carbon atoms. What do graphite and diamonds have in common. Diamond and graphite are both allotropes of carbon.
This makes graphite and diamonds allotropes of carbon along with amorphous which is commonly called soot or carbon black. This makes graphite and diamonds allotropes of carbon along with amorphous which is commonly called soot or carbon black. The chemical composition of the two is exactly the same.
As a result diamond is the ultimate abrasive whereas graphite is an excellent lubricant. Diamond can be yellow brown green less often blue black translucent white pink violet orange purple and red. They both have a giant covalent structure.

Fun Fact What Do Diamond Rings And Pencils Have In Common Fun Facts Facts Facebook Sign Up

Common Forms Of Carbon Including Amorphous Graphite Diamond Carbon Download Scientific Diagram

Which Pair Of Statements About Diamond And Graphite Is Correct

1 50 Explain How The Structures Of Diamond Graphite And C60 Fullerene Influence Their Physical Properties Including Electrical Conductivity And Hardness Tutormyself Chemistry

Different Allotropes Of Carbon Viz Graphite Diamond Fullerene And Download Scientific Diagram
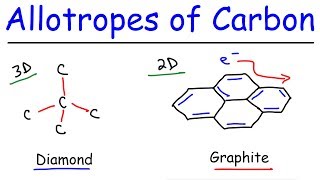
Allotropes Of Carbon Graphite Diamond Graphene Fullerenes Youtube

Quick Answer What Are The Similarities And Differences Between Graphite And Diamond Seniorcare2share

Why Diamond Is So Shiny Whereas Graphite Is Not Shiny Despite The Fact That Both Are Made Up Of Carbon Quora
Butterfly110 On Twitter How Can Graphite And Diamond Be So Different If They Are Both Composed Of Pure Carbon Bio110fall18 Nutmegsomething Https T Co D70j08szhv Twitter

Structure Of Diamond And Graphite Differences Similarities

1 Common Types Of Carbon A Coal B Graphite C Diamond D Download Scientific Diagram

Structure Of Diamond Vs Structure Of Graphite Gold Jewelry Simple Beautiful Diamond Earrings Boho Jewelry Diy

What Is The Link Between Diamond Graphite And Buckyballs Office For Science And Society Mcgill University
Open Knowledge Wiki What Is Graphite
What Is Common Between Graphite And Diamond Quora
What Are The Similarities And Differences Between Graphite And Diamond Quora
What Is The Geometric Structure Of Graphite Diamond And Buckyballs Quora

Why Diamond And Graphite Have Different Physical Properties But Same Chemical Properties What Is The Property Called Quora
Comments
Post a Comment